
Ligand Capture and Affinity Determination
Label-free methods for the measurement and characterization of biomolecular interactions are very popular and widely used, e.g. when conducting binding assays and screenings. Here, on-rates, off-rates, and affinities are determined based on an experimental set-up, in which a ligand is captured to a sensor’s surface; the ligand then interacts with an analyte that the sensor is probed with. Applied assay technologies comprise surface plasmon resonance (SPR), e.g. from BiacoreTM/Cytiva (formerly GE Healthcare), bio-layer interferometry (BLI), e.g. from FortéBio/Sartorius, and switchSENSE® from Dynamic Biosensors. The stable, uniform and oriented, i.e. site-directed, immobilization of the ligand is of considerable importance for the reliable determination of the binding kinetics of ligand to analyte.
Nano-CaptureLigands are optimized for the site-directed, specific, dense, and gentle immobilization of antibodies to biosensors. Conveniently, no antibody biotinylation is required. Their high selectivity enables the specific immobilization of antibodies, antibody fragments, and Fc-fusions. Because of their high affinity, they provide a stable baseline with negligible antibody dissociation. Nano-CaptureLigands’ chemical stability allows for a frequent regeneration for multiple reuse and the application of standard protocols for faster protocol development.
Besides Nano-CaptureLigands, which consist of biotinylated anti-immunoglobulin VHHs, also the biotinylated anti-GFP VHH/ Nanobody is a very valuable tool for capture of GFP-fusion proteins because of its extremely high 1 pM affinity, as determined by switchSENSE® technology. Note, also GST, MBP, and Spot VHHs and other Nanobodies from ChromoTek are very suitable for the immobilization of corresponding fusion proteins in biosensor assays.
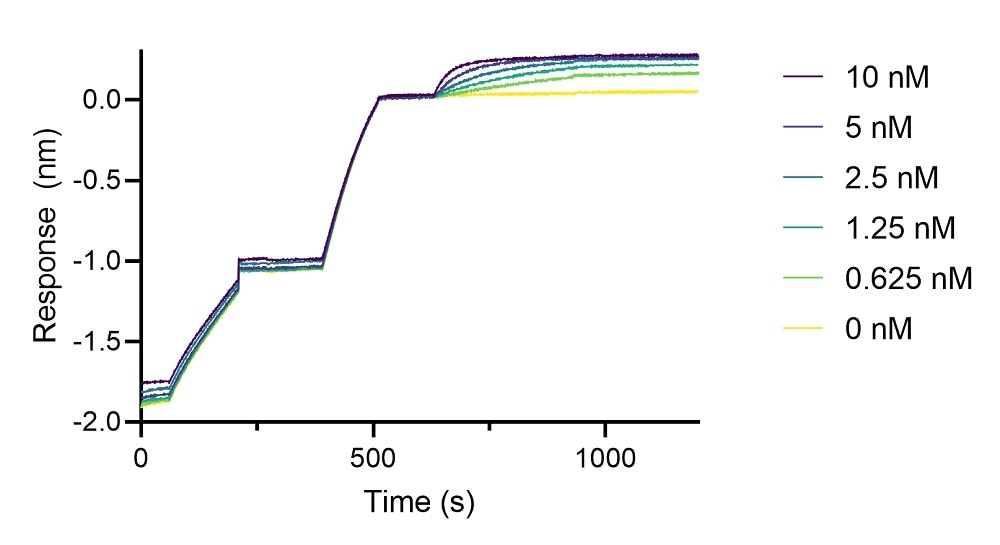
Biolayer interferometry (BLI binding kinetics of a human IgG1 anti-IgE antibody to human IgE.