
Spot Capture and Detection System
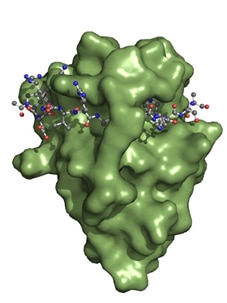
Interaction of Spot-Nanobody (green) with Spot Peptide. The soluble accessible surface of the Spot VHH is shown in green.
ChromoTek’s proprietary and novel Spot-System is the first peptide-tag specific Nanobody for universal capture & detection applications. It comprises the Spot-Tag®, an inert 12 amino acid peptide-tag (PDRVRAVSHWSS), and Spot-Nanobodies that specifically bind to Spot-tagged proteins with high affinity.
Nanobodies against peptide tags
In general, Nanobodies tend to bind to native, discontinuous conformational epitopes (3-dimensional epitopes) rather than to linear peptide epitopes (2-dimensional epitopes). However, ChromoTek has managed to develop exceptional anti-Spot-Tag Nanobody next to anti-Myc Nanobody and anti-V5 Nanobody, which recognize small linear peptide tags, respectively.
Applications
- Immunoprecipitation (IP) / Co-IP: Spot-Trap
- Co-IP/MS: Spot-Trap and iST Spot-Trap Kit
- Protein purification: Spot-Cap
- Immunofluorescence: Spot-Label
- Western Blotting: Spot-tag antibody [28a5]
Introduction and binding mechanism
The Spot Nanobody, also termed Spot VHH, is derived from single-domain alpaca antibody fragments. The Spot Nanobody recognizes the Spot-Tag® sequence motif PDRVRAVSHWSS. Binding occurs when the Spot-Tag peptide is embedded on the surface of the Spot Nanobody and acts like a β-sheet extension of the Spot-VHH. Specificity is determined by interactions of the Spot-Nanobody’s side chains to the Spot-Tag peptide. Notably, the bound Spot-peptide is also clamped by two amino acid side chains of the Spot-Nanobody. This binding mechanism explains the high affinity of the Spot-Nanobody.
Spot-Nanobody is fully validated
- Recombinantly produced for constant high quality
- Sequence known
- Structure determined
- Binding mechanism understood
Spot-Nanobody is better performing in many capture and detection applications than other antibodies. Most of these antibodies are conventional IgGs, which may suffer from stability and size. Spot-Nanobody however is small size to better access the Spot-Tag epitope. In addition, Spot-Nanobody is very stable and binds to Spot-Tag even under harsh conditions. Non-IgG type affinity reagents for affinity-tags can’t be used for detection and imaging of tagged proteins, whereas Spot-Nanobody has an outstanding performance in immunofluorescence and Western blotting. Spot-Nanobody is the first Nanobody/ peptide-tag that was applied in super-resolution microscopy.
Key Features
- Small size of just 14.7 kDa or 2 nm
- High affinity binding of Spot-Tag with KD = 0.7 nM
- High chemical and thermal stability
with Tm = 65.1°C
- No contaminating heavy & light antibody chains
- Native elution with Spot-peptide & by pH shift
- Matrix can be regenerated
- Well characterized
- Optimized Nanobody for gentle elution at 4°C
Key Benefits
- Specific binding with low background
- Robust handling and stable in harsh conditions
- Native & non-native elution
- Highly specific binding
- Higher resolution
- Good epitope access & sample penetration
- Good detection limits
- Validated
- Effective purification of Spot-tagged proteins
Affinity Optimized Short Peptide Tag
The Spot-Tag® has the affinity-optimized 12 amino acid sequence PDRVRAVSHWSS. Spot-Nanobodies bind strongly to the Spot-Tag. The inert tag sequence can be added to the N-terminal and to the C-terminal side of the protein of interest. This works by PCR using modified primers or by using any of the Spot vectors offered (click here for more information). The expression of Spot-tagged proteins has been successfully tested in bacteria, yeast, mammalian cell lines, plant and insect cells.
Spot-Nanobody advantages compared to other antibodies:
- More robust & stable than IgG antibodies
- High affinity of 6 nM to Spot-tagged proteins and 0.7 nM to Spot-Tag
- Less background than other tested affinity resins
- Less unspecific binding than other tested capture and detection antibodies
- Higher resolution than conventional antibodies
- Better epitope accessibility and tissue penetration than conventional IgG antibodies
- Effective and gentle elution at 4°C (Spot-Cap)
Spot-Tag advantages compared to other peptide tags:
- Less negatively charged than FLAG®-, Myc-, and HA-tags
- Short
- Inert, unstructured tag does not interference with fusion protein structure and function
- Binding of a single tag
- Suitable for ubiquitination assays: comprises no lysine