
Live Cell Imaging
Live-cell imaging allows the visualization of cellular processes using time-lapse microscopy. Structural changes and physiological processes can be observed in real-time. Other common microscopy techniques such as immunofluorescence usually require cell fixation and permeabilization, which shows only a snapshot of the cells at a certain timepoint and might lead to artefacts. In contrast, during live-cell imaging, dynamic changes can be analyzed, particularly, when subcellular structures are being investigated.
Probes for live-cell imaging comprise fluorescent proteins, peptides, organic, and inorganic fluorophores (quantum dots). Obviously, it is crucial for live-cell imaging to shuttle the fluorescent probes inside the cell under conditions that still allow cell viability and growth. Especially for macromolecules like proteins and most peptides this remains a challenge. ChromoTek has overcome this issue by the proprietary Chromobody® technology for live-cell imaging: Chromobodies are Nanobodies genetically fused to a fluorescent protein (GFP or RFP) and are intracellularly expressed. They are suitable for a great variety of eukaryotic cells, ranging from plants to vertebrates. Within the living cell, the Nanobody is able to bind to its target protein and thereby marks it with the fluorescent protein. Plasmids encoding for Chromobodies can be transiently transfected into cells, but also stable cell lines or even transgenic organisms with constitutive or responsive Chromobody expression can be generated.
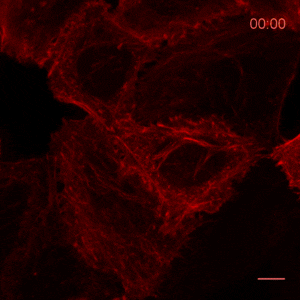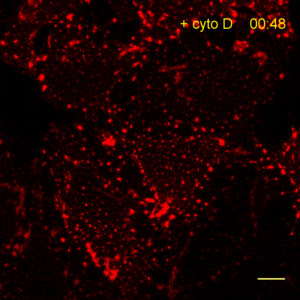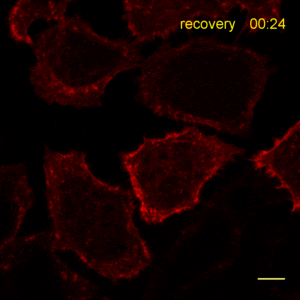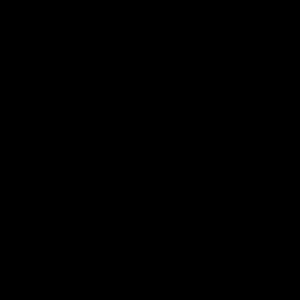
The time lapse video shows reorganization of actin filaments after treatment with Cytochalasin. For details see Actin-Chromobody.
| Product | Formats (Product citations in parentheses) |
|---|---|
| Actin-Chromobody® | TagGFP2 (15), TagRFP (5) |
| Nuclear Actin-Chromobody® | TagGFP2 |
| Cell Cycle-Chromobody® | TagRFP (11) |
| Dnmt1-Chromobody® | TagGFP2, TagRFP (1) |
| Histone-Chromobody® | eGFP (1) |
| Lamin-Chromobody® | TagGFP2 (5) |
| PARP1-Chromobody® | TagGFP2, TagRFP (2) |
| Vimentin-Chromobody® | TagGFP (2) |