
Affinity Purification
Affinity purification is a technology that enriches or purifies a protein from a complex mixture of biomolecules such as a cellular lysate, cell culture supernatant or serum etc. The protein of interest is selectively bound by a ligand like an antibody or Nanobody, which is attached to a matrix, such as agarose beads.
Frequently, the protein of interest is expressed recombinantly and fused to a protein- or peptide-tag, which is bound specifically and reversibly by the affinity resin. Enriched protein can be released either by competitive elution using a molecule like a peptide competing for binding to the affinity resin, or simply by changing the buffer conditions, such as pH shift or change in ionic strength.
Most commonly, affinity purification is done either as batch purification or via a column packed with the affinity resin (affinity chromatography).
Nanobodies are especially well suited for affinity purification, as they combine both high selectivity with a high degree of robustness. Since Nanobodies are very stable and remain functional even under extreme buffer conditions, highly stringent washing protocols (e.g. detergent, low pH, high salt etc.) and multiple regeneration cycles of the affinity resin can be applied. Accordingly, they are perfect tools for affinity purification.
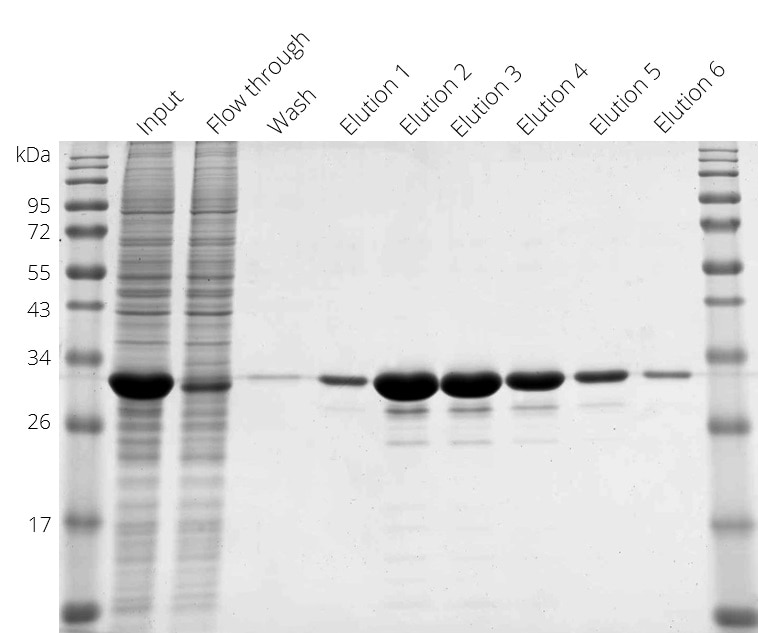
Purification of Spot-tagged protein. Elution with Spot-peptide at 4°C.